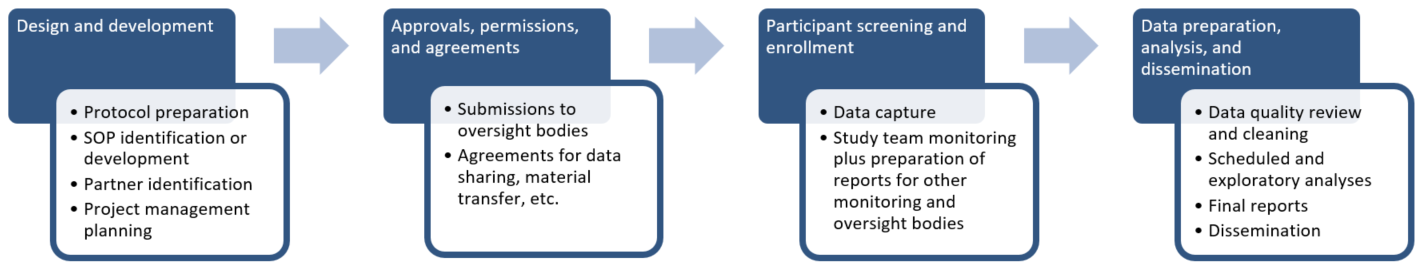
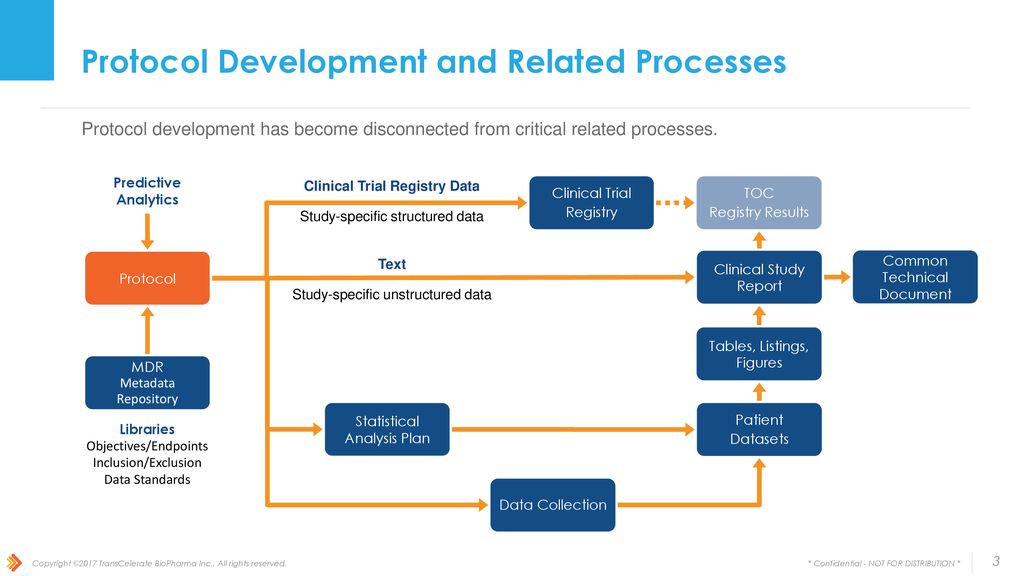
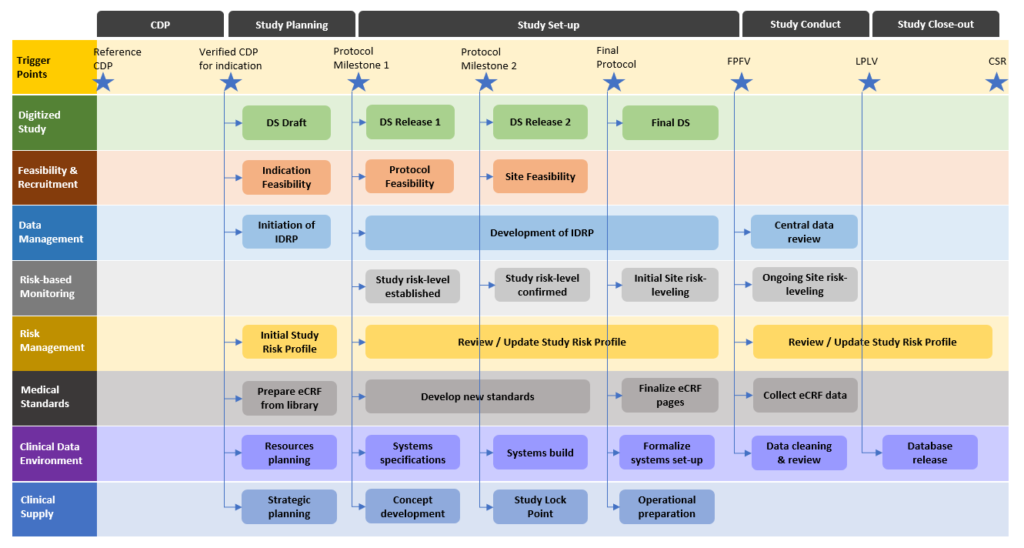
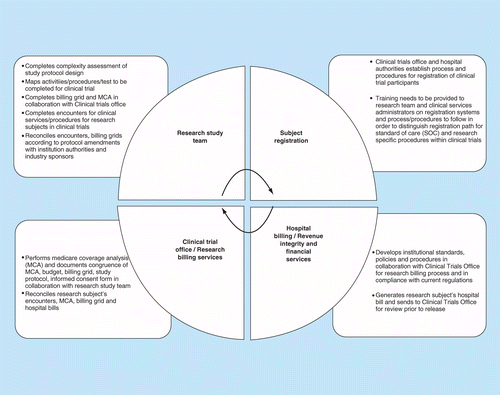
Protocol-in-a-Day Workshop: A Lean Approach to Clinical Trial Development and Focus on Junior Faculty Development - Advances in Radiation Oncology

PPT - The Clinical Research Nurse as an expert resource in the protocol development and implementation of a research study – a PowerPoint Presentation - ID:380433
Optimization of protocol design: a path to efficient, lower cost clinical trial execution | Future Science OA

11/11/04 Clinical Research and Development in the Pharmaceutical and Biotechnology Industry Robert Anderson, MHA, CCRA, CCRCP Director, Clinical Trials. - ppt download

Improving outcome reporting in clinical trial reports and protocols: study protocol for the Instrument for reporting Planned Endpoints in Clinical Trials (InsPECT) | Semantic Scholar

The Evolution of Master Protocol Clinical Trial Designs: A Systematic Literature Review - ScienceDirect

Incorporating Site-less Clinical Trials Into Drug Development: A Framework for Action - Clinical Therapeutics
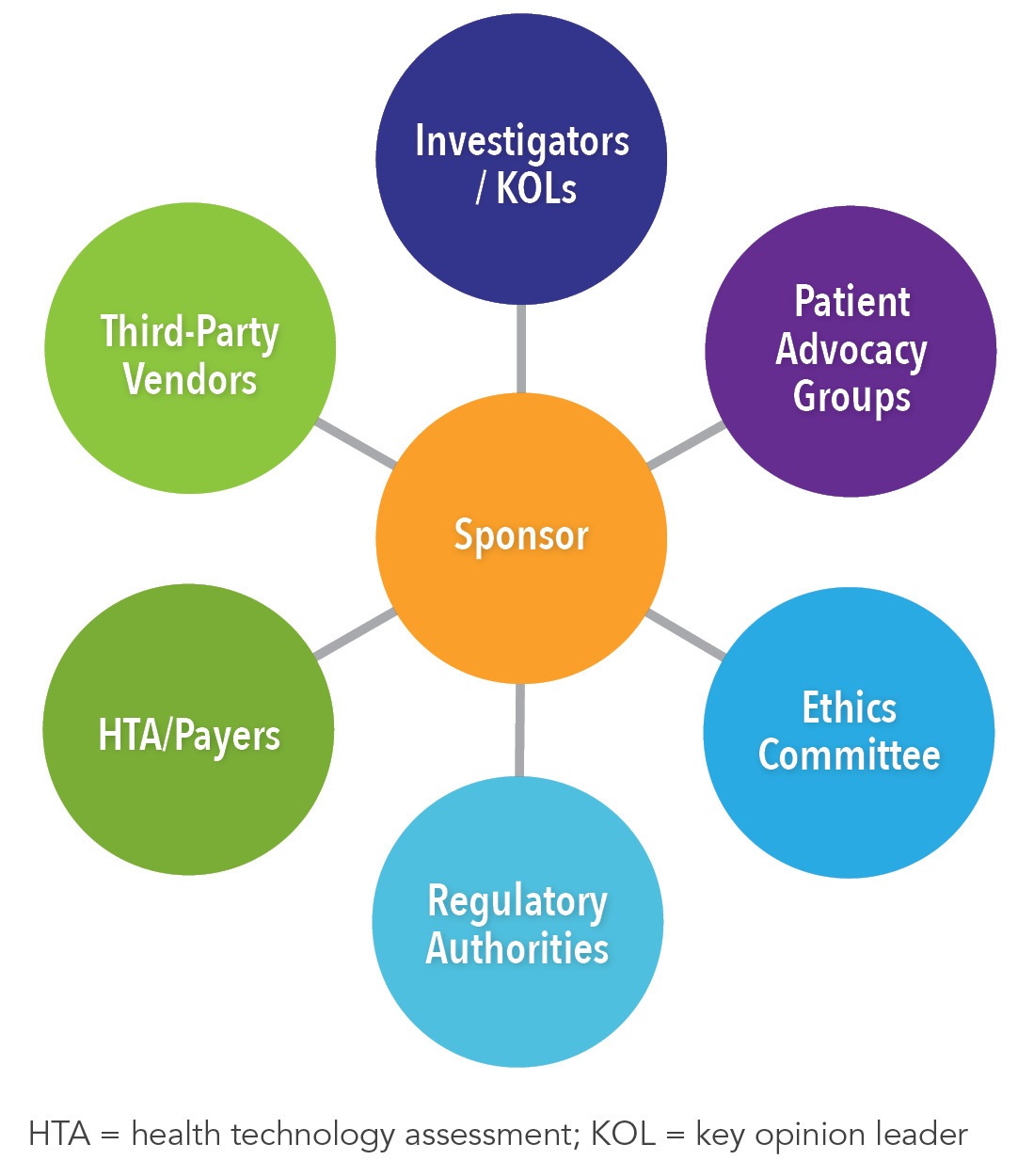
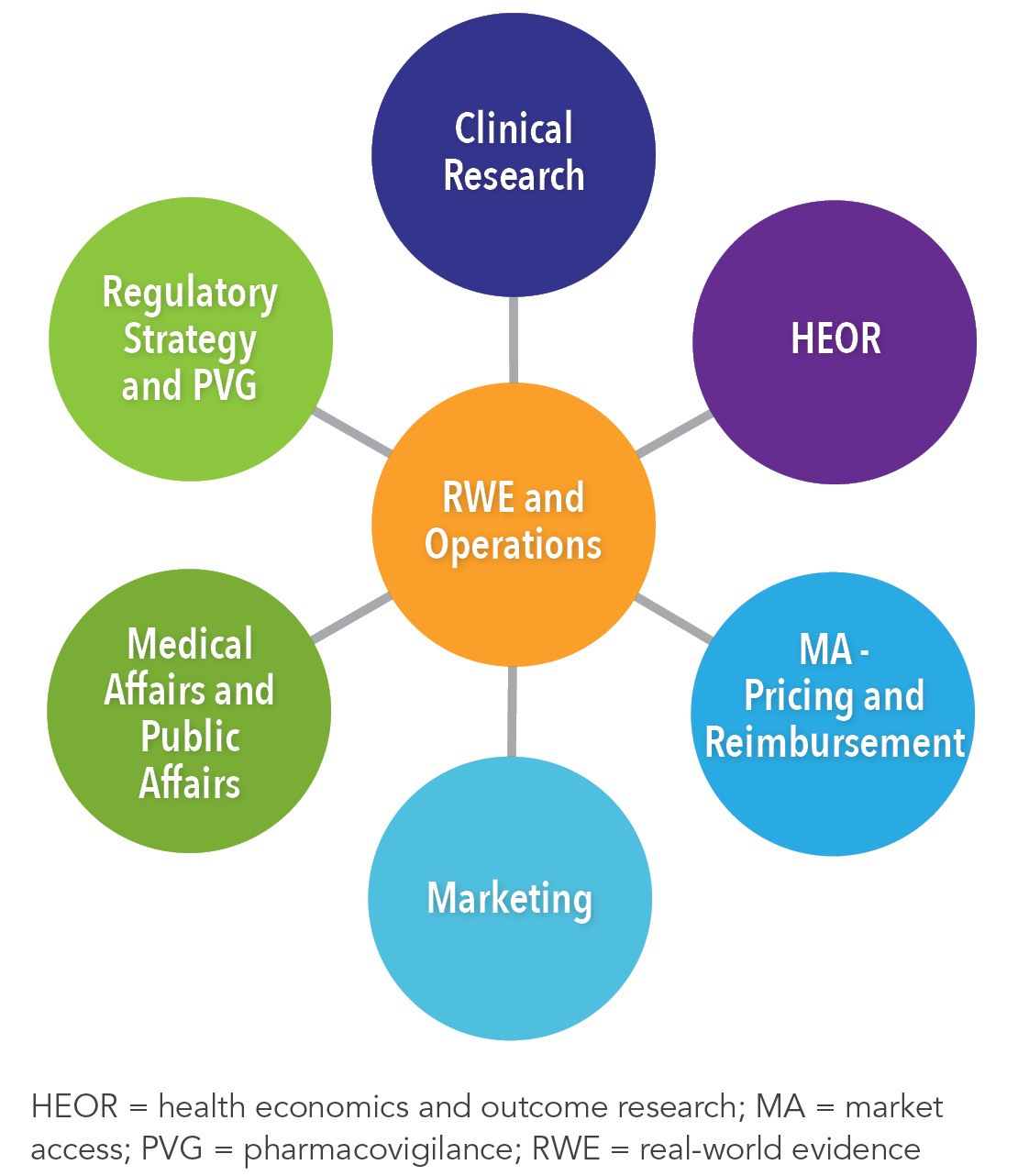
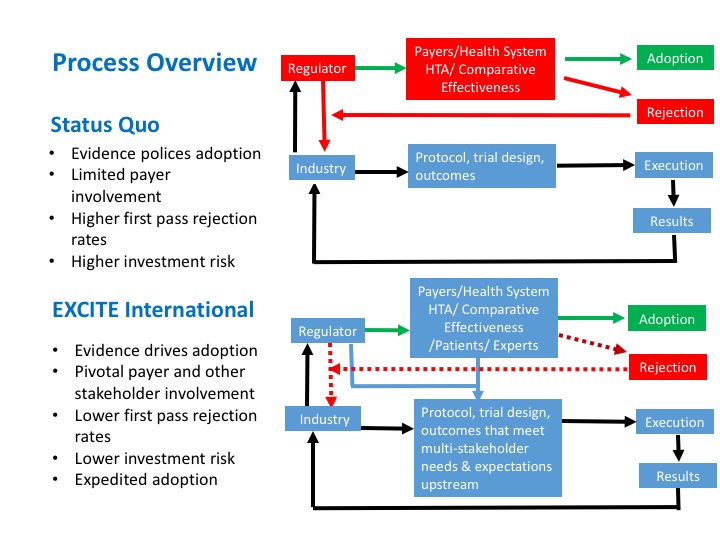
White Paper: Protocol Design in Real-World Evidence: The Indispensable Link Between Strategic Need and Study Execution - Evidera