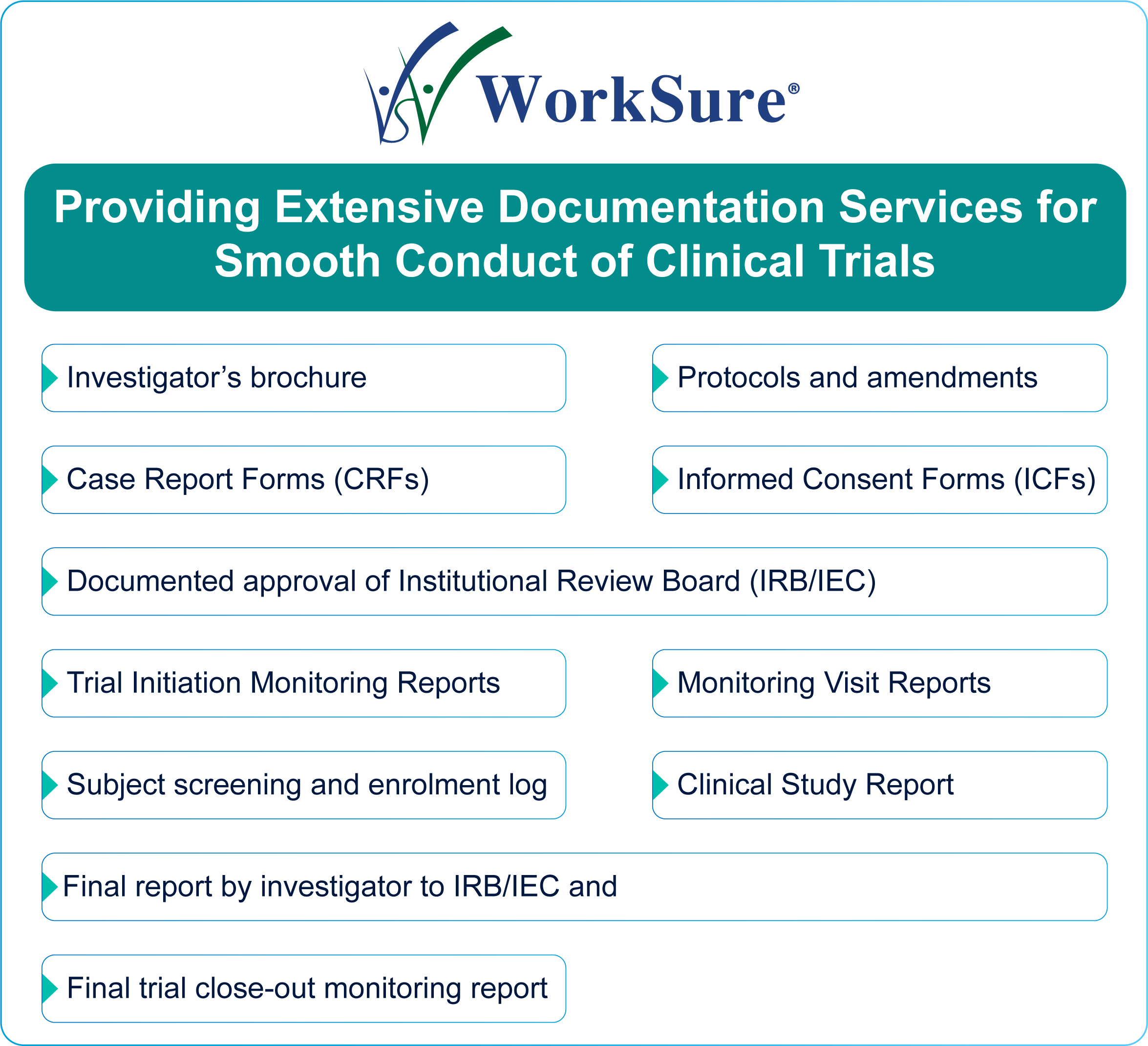
ICH GCP - 8. Essential documents for the conduct of a clinical trial: ICH E6 (R2) Good clinical practice

Essentail Documents For Conduct of A Clinical Trial | PDF | Institutional Review Board | Clinical Trial

Research2note on Twitter: "The what, when and why of research essential documents @NHSRDForum @dollyblue3 @DigitalCRN @DarkNatter @Research_Innov @WeNurses https://t.co/yPlmOQEHc6" / Twitter
Nova Southeastern University Standard Operating Procedure for GCP Title: Essential Documents for a Clinical Trial Version # 1 S
Abbreviated List of Essential Study Documents for a Clinical Trial at Providence Health Care Essential Documents are those docum

Table 1 from Time-Sinks during the Planning Phase of Investigator Initiated Trials (IITs).Results of an Online Survey | Semantic Scholar