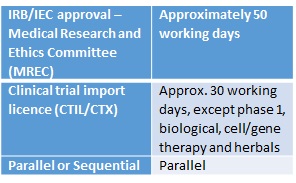
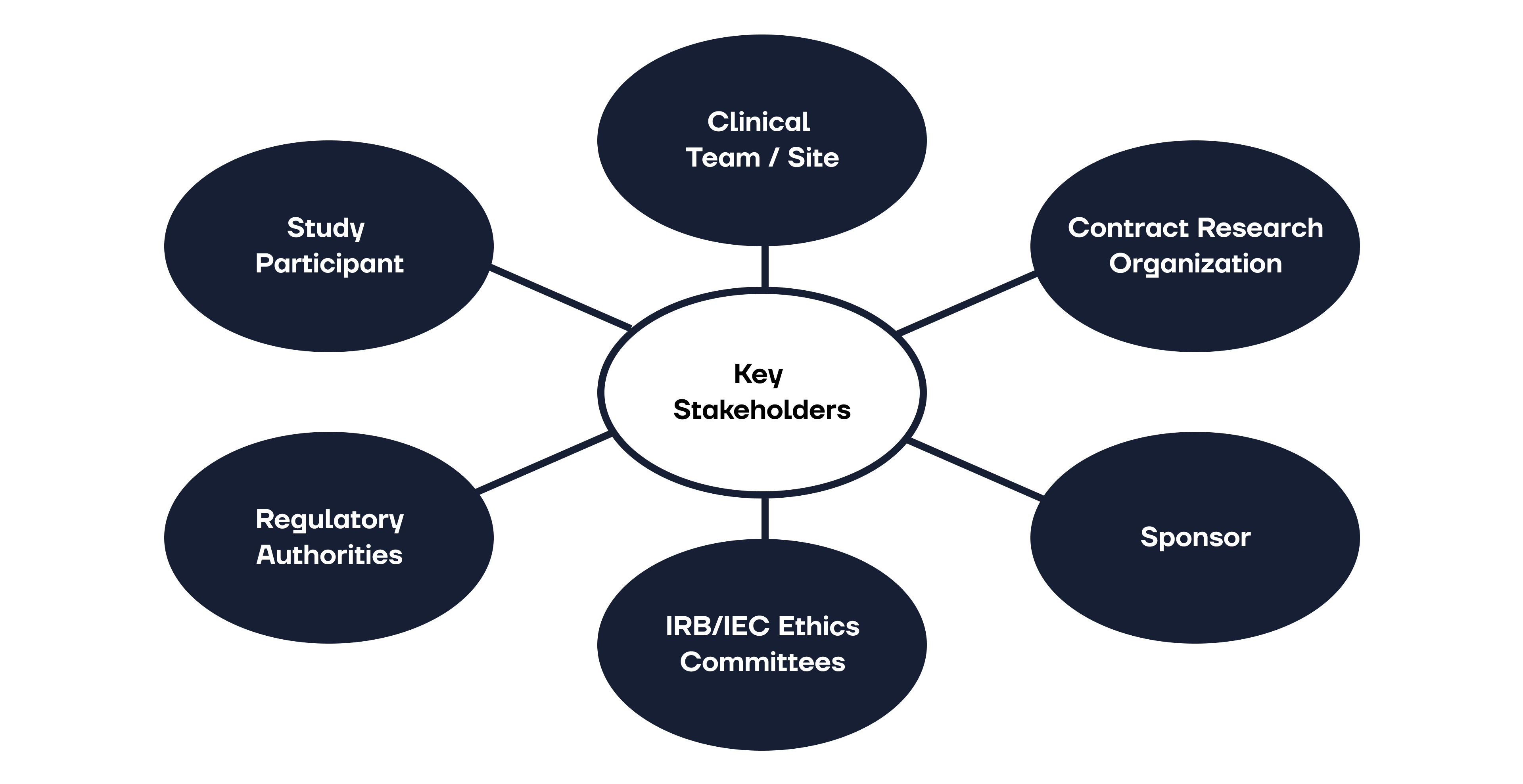
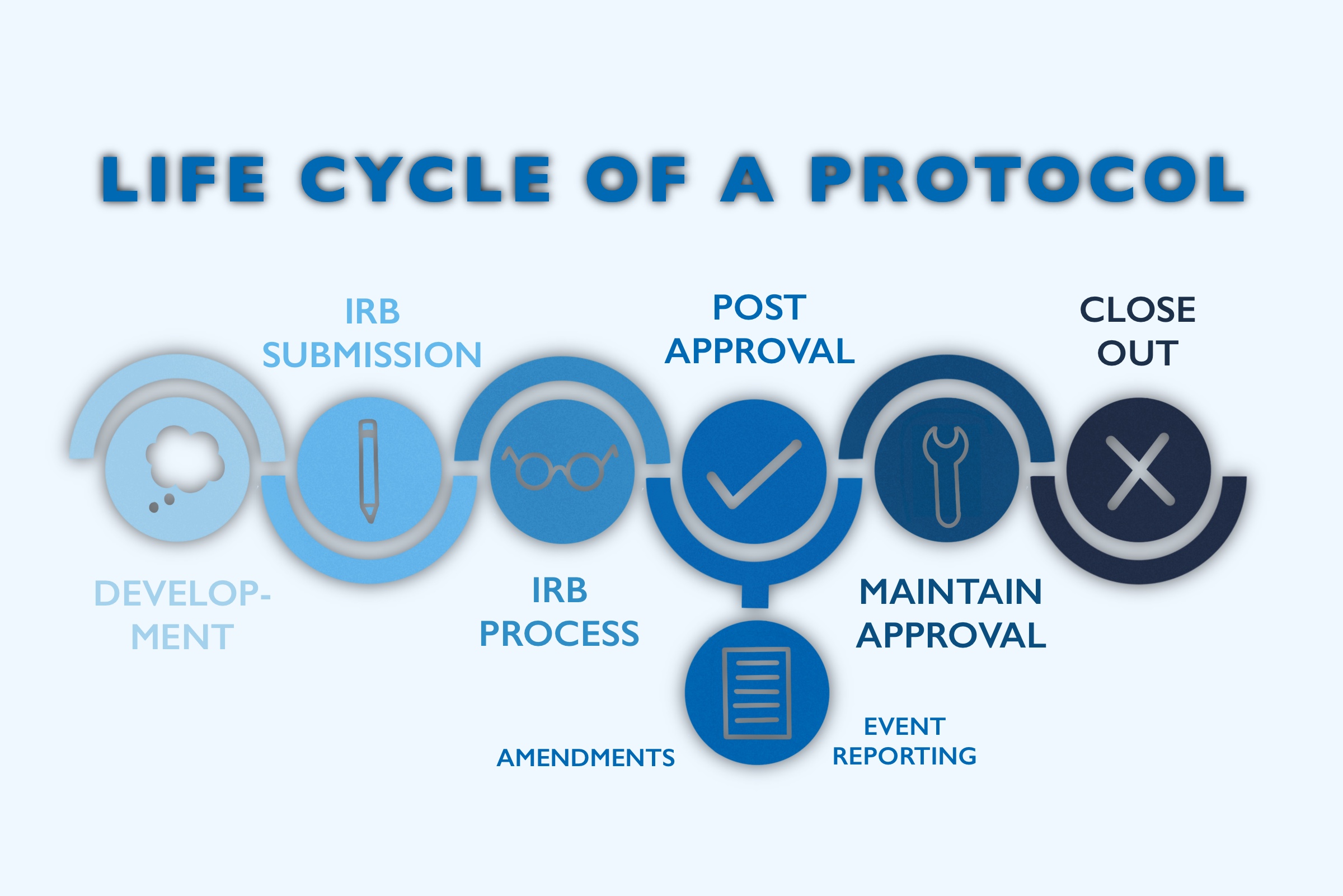
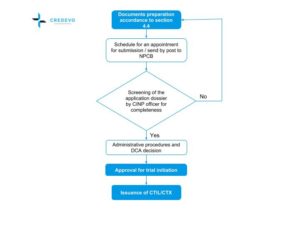
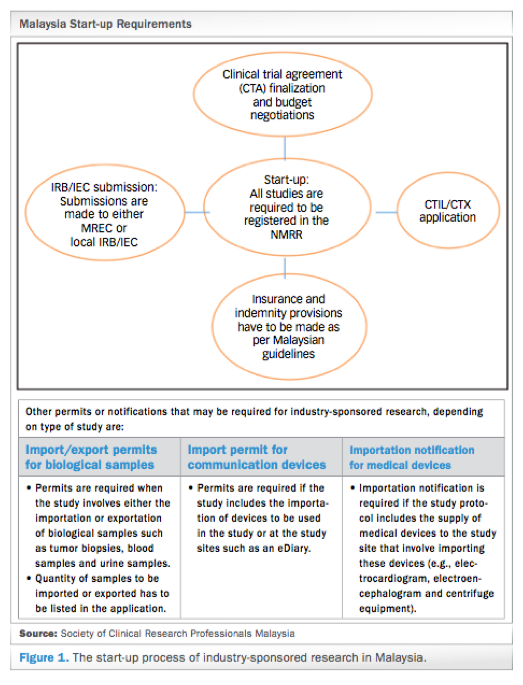
ICH GCP - 3. INSTITUTIONAL REVIEW BOARD/INDEPENDENT ETHICS COMMITTEE (IRB/ IEC): ICH E6 (R2) Good clinical practice
![What is an Institutional Review Board (IRB/IEC)? [Video] - LearnGxP: Accredited Online Life Science Training Courses What is an Institutional Review Board (IRB/IEC)? [Video] - LearnGxP: Accredited Online Life Science Training Courses](https://learngxp.com/wp-content/uploads/2018/06/ELM-901-09-What-is-an-Institutional-Review-Board-IRB-IEC_.png)
What is an Institutional Review Board (IRB/IEC)? [Video] - LearnGxP: Accredited Online Life Science Training Courses

What is an IRB? And Why Should I Care? - DOCUMENT TRANSLATION, AUDIO TRANSCRIPTION, & PHONE INTERPRETATION

IRB IEC board - responsibilities ,composition, functions and operations, procedures and records - Studocu
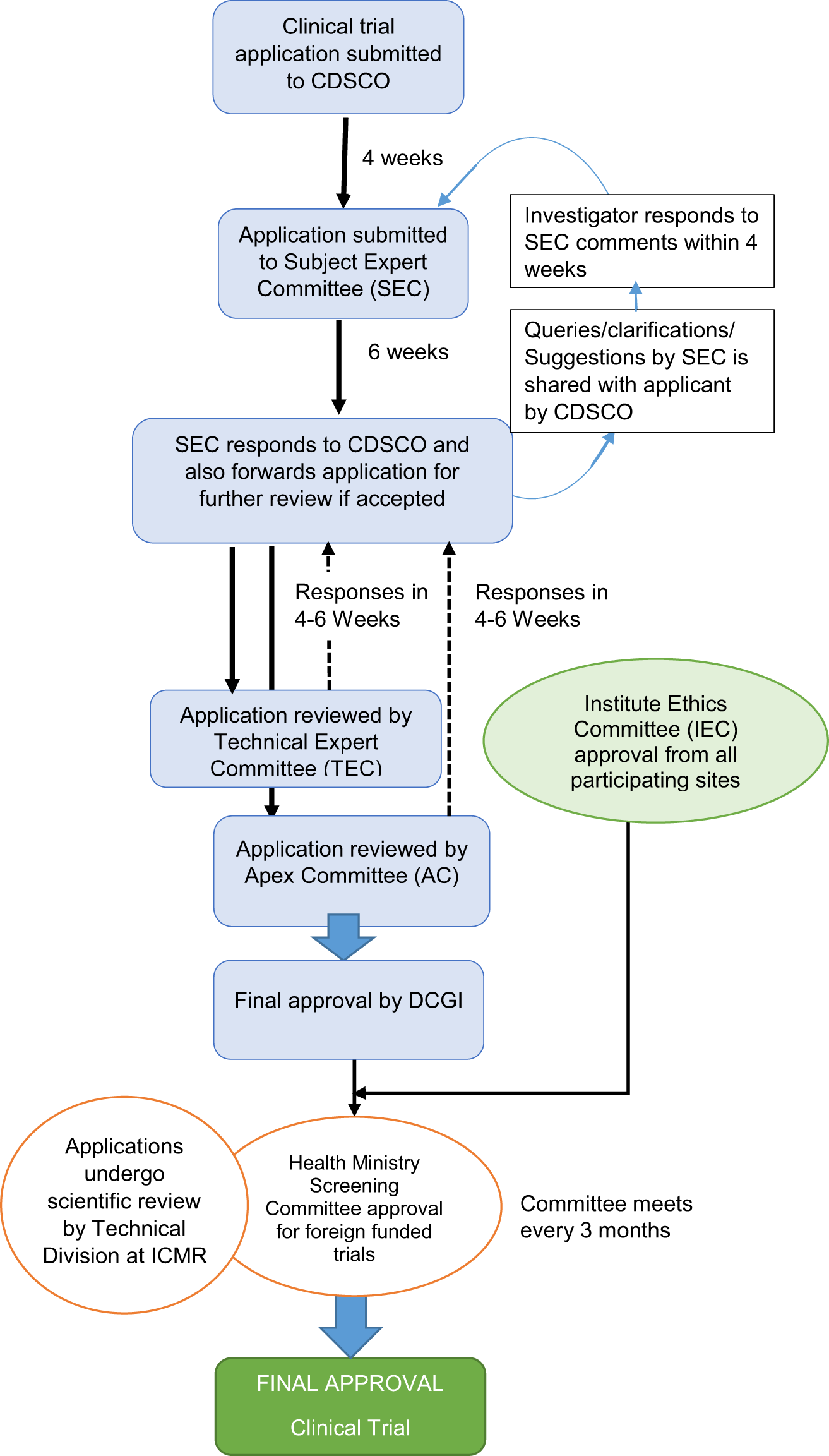
Issues, challenges, and the way forward in conducting clinical trials among neonates: investigators' perspective | Journal of Perinatology






![PDF] Institutional Review Boards and Independent Ethics Committees | Semantic Scholar PDF] Institutional Review Boards and Independent Ethics Committees | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/057fccd15f43969114b301ea374eaaf0bcb9caa1/7-Table8.3-1.png)