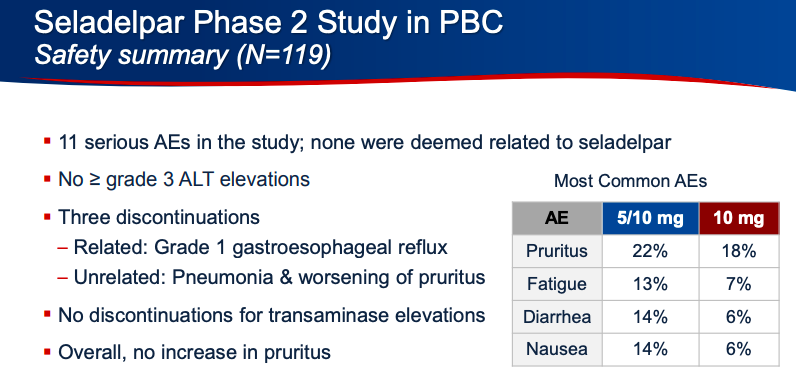
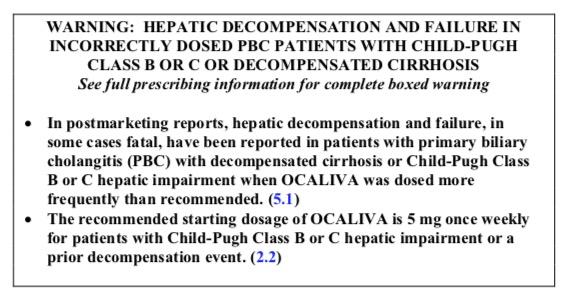
Intercept Pharmaceuticals Stock Falls After FDA Warns Of Ocaliva-Related Deaths | Stock News & Stock Market Analysis - IBD
FDA's black box warning – Stock Market Research, Option Picks, Stock Picks,Financial News,Option Research
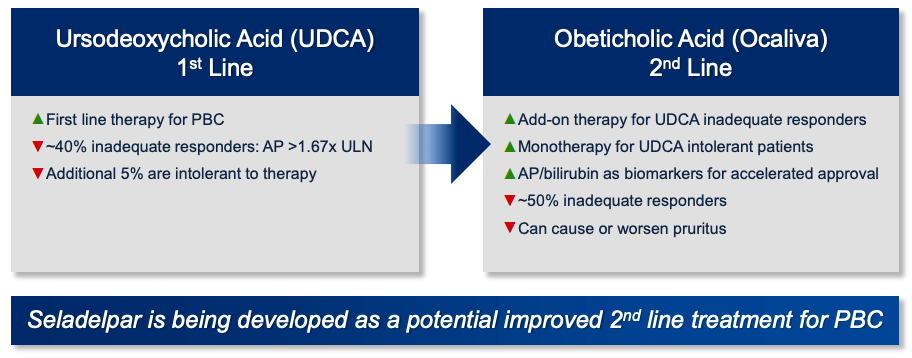
Intercept: Ocaliva Woes Extend Far Beyond Itching And Blackbox Warnings (NASDAQ:ICPT) | Seeking Alpha